When the deadly Ebola disease was ravaging human lives in West Africa, the National Institutes of Health (NIH) of the United States sponsored a randomized control trial of vaccines that researchers hoped might prevent the deadly disease in patients.
Some British medical research bodies also took part in the programme. The testing took place in one of the worse affected countries, Liberia with 600 patients. The plan was to extend it to about 30,000 patients.
Although the Liberian government for whatever reason claimed that volunteers in the testing were not paid for participating, the BuzzFeed news later had an email from the National Institute of Allergy and Infectious Disease, confirming that participant received “$40 in cash for their first visit, which involves an injection and a blood draw.”
Dramatically, as the trials were going on, one drug manufacturer from the US, Chimerix which trades on NASDAQ as CMRX announced it was pulling out of the trial. Chimerix also announced that it would not participate in “any future trials” of brincidofovir as an Ebola treatment. This shocked many observers and partners who want to find a cure to the disease. Chimerix was behind the drug called brincidofovir. Brincidofovir was meant to treat those who have been affected by Ebola.
Although some mainstream media decided to act as the mouthpiece of Chimerix by reporting that the company pulled out because there were no more new patients on which to test the drug, that turned out not to be true. Even as we speak today, the disease is still being recorded but just that the number has reduced. Therefore, to say that there were no more new patients to try the drug was highly inaccurate. The British team involved in the testing programmed even called Chimerix’s decision to pull out “a bit abrupt.”
Chimerix spokesman, Joseph Schepers will later tell the BuzzFeed news that the decision to withdraw from the trial was made after discussions with “the Food and Drug Administration (FDA) and other federal agencies, as well as international regulatory and public health groups, and against the backdrop of a rapidly diminishing pool of patients with Ebola Virus Disease in Liberia.”
However, the BuzzFeed after publishing this interview with Joseph Schepers received an instant correction from the FDA. The FDA claimed it played no oversight role in the trial and could not be concerned whether a company pulls out or not.
“The FDA did not approve the trial. The FDA evaluates proposed clinical trials when sponsors seek permission to conduct such trials by submitting investigational new drug applications,” said the FDA’s Dr. Luciana Borio.
As Chimerix will not reveal the actual reason it pulled out from the trial, but will rather play hide and seek with the public, the BuzzFeed News decided to dig deep into the story.
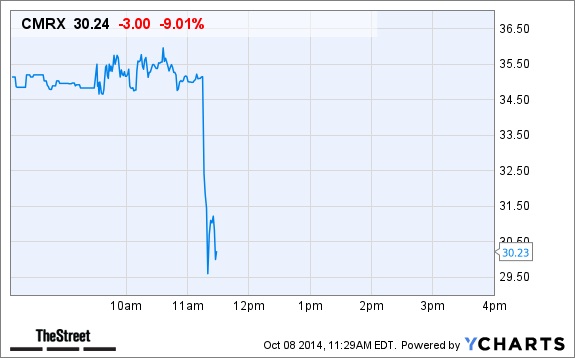
And it emerged that Chimerix had raised $122 million for further research with the drug. But the Ebola disease brought volatility to its share value. In October 2014, a Liberian citizen, Thomas Duncan died of Ebola in Texas hospital after traveling from the West African country to the US. Duncan was the first person to test positive of Ebola on US soil. Doctors had treated Duncan with brincidofovir, hoping that he will survive.
Brincidofovir was originally developed as a treatment for small pox. The US Department of Health and Human Services supported the development of the drug with $103 million. And in the wake of the Ebola outbreak, the plan was to try the drug so that when it succeeds in curing the disease, the company and of course the government makes a windfall out of it.
But hours after the announcement of the death of Duncan, Chimerix stock prices reportedly fell by 11%. That was a huge loss. And as we all know by now that the Pharma companies are interested in profit and not helping patients, Chimerix exhibited that. They were in West Africa for money but now that they are losing money, it made no sense for them to continue staying in West Africa. That is very simple to understand.
You want to support Anonymous Independent & Investigative News? Please, follow us on Twitter: Follow @AnonymousNewsHQ
This Article (Revealed: Why A US Drug Manufacturer Abandoned Ebola Testing Programme In West Africa) is free and open source. You have permission to republish this article under a Creative Commons license with attribution to the author and AnonHQ.com