
Written by: Simon Cheong
Who determines whether chemicals are safe — and why do different governments come up with such different answers?
In the United States, children can drink fruit juice beverages made with Red Dye No. 40 and eat macaroni and cheese colored with Yellow Dye No. 5 and No. 6. Yet in the U.K., these artificial colorings have been taken off the market due to health concerns, while in the rest of Europe, products that contain them must carry labels warning of the dyes’ potential adverse effect on children’s attention and behavior. 
Atrazine, which the U.S. Environmental Protection Agency says is estimated to be the most heavily used herbicide in the U.S., was banned in Europe in 2003 due to concerns about its ubiquity as a water pollutant. Also widely used by U.S. farmers are several neonicotinoid pesticides that the European Commission says pose “high acute risks” to bees and has placed under a two-year moratorium. These pesticides — with which about 90 percent of the corn planted in the U.S. is treated — have been identified in numerous scientific studies as toxic to bees and are considered likely contributors to the alarming global decline of these essential pollinators.
The U.S. Food and Drug Administration places no restrictions on the use of formaldehyde or formaldehyde-releasing ingredients in cosmetics or personal care products. Yet formaldehyde-releasing agents are banned from these products in Japan and Sweden while their levels — and that of formaldehyde — are limited elsewhere in Europe. In the U.S., Minnesota has banned in-state sales of children’s personal care products that contain the chemical.
Use of lead-based interior paints was banned in France, Belgium and Austria in 1909. Much of Europe followed suit before 1940. It took the U.S. until 1978 to make this move, even though health experts had, for decades, recognized the potentially acute — even deadly — and irreversible hazards of lead exposure. 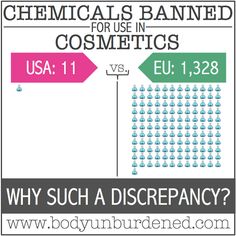
These are but a few examples of chemical products allowed to be used in the U.S. in ways other countries have decided present unacceptable risks of harm to the environment or human health. How did this happen? Are American products less safe than others? Are Americans more at risk of exposure to hazardous chemicals than, say, Europeans?
Not surprisingly, the answers are complex and the bottom line, far from clear-cut. One thing that is evident, however, is that “the policy approach in the U.S. and Europe is dramatically different,” says Stacy Malkan, co-founder of the Campaign for Safe Cosmetics.
An Ounce of Precaution
A key element of the European Union’s chemicals management and environmental protection policies — and one that clearly distinguishes the EU’s approach from that of the U.S. federal government — is what’s called the precautionary principle.
This principle, in the words of the European Commission, “aims at ensuring a higher level of environmental protection through preventative” decision-making. In other words, it says that when there is substantial, credible evidence of danger to human or environmental health, protective action should be taken despite continuing scientific uncertainty.
In contrast, the U.S. federal government’s approach to chemicals management sets a very high bar for the proof of harm that must be demonstrated before regulatory action is taken.
This is true of the U.S. Toxic Substances Control Act, the federal law that regulates chemicals used commercially in the U.S. The European law regulating chemicals in commerce, known as REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), requires manufacturers to submit a full set of toxicity data to the European Chemical Agency before a chemical can be approved for use. U.S. federal law requires such information to be submitted for new chemicals, but leaves a huge gap in terms of what’s known about the environmental and health effects for chemicals already in use. Chemicals used in cosmetics or as food additives or pesticides are covered by other U.S. laws — but these laws, too, have high burdens for proof of harm and, like TSCA, do not incorporate a precautionary approach.
Same Study, Different Conclusions
What does this mean in practice? In the case of Red Dye No. 40, Yellow Dye No. 5 and Yellow Dye No. 6, it means that after considering the same evidence — a 2007 double-blind study by U.K. researchers that found that eating artificially colored food appeared to increase children’s hyperactivity — European and U.S. authorities reached different conclusions. In the U.K., the study persuaded authorities to bar use of these dyes as food additives. The EU chose to require warning labels on products that contain them — greatly reducing their use, according to Lisa Lefferts, senior scientist with the nonprofit Center for Science in the Public Interest in Washington, D.C. In the U.S., the study prompted the CSPI to petition the Food and Drug Administration for a ban on a number of food colorings. But in its review of these dyes, presented in 2011, the FDA found the study inconclusive because it looked at effects of a mixture of additives rather than individual colorings — and so these colors remain in use.
While FDA approval is required for food additives, the agency relies on studies performed by the companies seeking approval of chemicals they manufacture or want to use in making determinations about food additive safety, Natural Resources Defense Council senior scientist Maricel Maffini and NRDC senior attorney Tom Neltner note in their April 2014 report, Generally Recognized as Secret. “No other developed country that we know of has a similar system in which companies can decide the safety of chemicals put directly into food,” says Maffini. The standing law that covers these substances — the 1958 Food Additives Amendment to the 1938 Federal Food, Drug and Cosmetic Act — “makes requiring testing [of chemicals] more cumbersome than under TSCA,” says Neltner.
The two point to a number of food additives allowed in the U.S. that other countries have deemed unsafe. Among these are “dough conditioners,” additives to enhance flour’s strength or elasticity. The International Agency for Research on Cancer considers one such chemical, potassium bromate, a possible carcinogen. This has led the EU, Canada, China, Brazil and other countries to ban its use. Although the FDA limits the amount of these compounds that can be added to flour and has urged bakers to voluntarily discontinue their use, it has not banned them. Earlier this year, the sandwich chain Subway made headlines by announcing it would discontinue using the dough conditioner azodicarbonamide, which is approved by the FDA but whose breakdown products have raised health concerns. 
Do-It-Yourself Decision Making
Reliance on voluntary measures is a hallmark of the U.S. approach to chemical regulation. In many cases, when it comes to eliminating toxic chemicals from U.S. consumer products, manufacturers’ and retailers’ own policies — often driven by consumer demand or by regulations outside the U.S. or at the state and local level — are moving faster than U.S. federal policy. On June 3, the California-based health-care company Kaiser Permanente announced that all its new furniture purchases — worth $30 million annually — would be free of chemical flame retardants. The same day, Panera Bread announced that the food served in its 1,800 bakery-cafés would be free of artificial additives by the end of 2016. Any number of large manufacturing companies and retailers — Nike, Walmart, Target, Walgreens, Apple and HP to name but a few — have policies barring chemicals from their products that U.S. federal law does not restrict.
This is also true of a number of cosmetic ingredients — for example, chemicals used in nail polish. After the EU banned a plasticizer called dibutyl phthalate from nail polish due to concerns over potential endocrine-disrupting and other adverse health effects in 2004, many global brands changed their ingredients. So while the FDA has not issued a regulation on its use, DBP is now found in fewer nail cosmetics sold in the U.S. In fact, the FDA actually bars only a specific handful of ingredients from cosmetics due to their toxicity.
“Cosmetics regulations are more robust in the EU than here,” says Environmental Defense Fund health program director Sarah Vogel. U.S. regulators largely rely on industry information, she says. Industry performs copious testing, but current law does not require that cosmetic ingredients be free of certain adverse health effects before they go on the market. (FDA regulations, for example, do not specifically prohibit the use of carcinogens, mutagens or endocrine-disrupting chemicals.) So, even though the personal care products and cosmetics products industry has extensive voluntary ingredient safety guidelines — and obvious incentives to meet them — they are not legal requirements.
Warnings, Advisories and Voluntary Phase-outs
Also worth noting is that U.S. laws regulating chemical use in food and cosmetics were first developed to protect American consumers from being sold “adulterated,” mislabeled or otherwise dishonestly marketed products — rather than with an eye on toxicity (though the two goals often coincide). The law continues to work along those lines. For example, when certain hairstyling products were found to contain formaldehyde or formaldehyde-releasing agents at levels causing health problems for salon workers, the FDA issued a warning saying that the products should be labeled (either on the product container or company website) with an appropriate caveat about the products’ potential health hazards. As a result, despite ample scientific evidence about adverse respiratory health effects of formaldehyde exposure and that formaldehyde is a skin irritant and potential occupational carcinogen, these hairstyling products continue to be sold in the U.S.
For the FDA to restrict a product or chemical ingredient from cosmetics or personal care products involves a typically long and drawn-out process. What it does more often is to issue advisories — as it has recently for the antibacterial ingredient triclosan, which is used in many soaps. In the meantime, based on growing scientific evidence of problematic health and environmental impacts — and indications that triclosan may not make hand-washing more effective — a number of manufacturers, among them Johnson & Johnson and Procter & Gamble, decided to eliminate the ingredient from their products. This spring, Minnesota became the first state to legally restrict its use.
The process for restricting chemical use under TSCA can also take years; in fact, only a handful of chemicals have ever been barred under TSCA. Instead, the Environmental Protection Agency, which administers TSCA, often works with companies on voluntary phase-out programs — which also take years to complete — as it has with the flame retardants known as polybrominated diphenyl ethers or PBDEs.
Meanwhile, U.S. companies manufacturing products that range from electronics to office products, sports gear, automobile parts and trendy clothing have been following the emerging science — along with international regulations, local policy and consumer demand — and developing policies and products that eliminate use of chemicals with well-documented hazards. While these voluntary efforts are resulting in products that contain fewer chemicals of concern, they do have limitations. One is transparency: Companies don’t always fully disclose such policy details. Another is that such policies don’t cover all products on the market, leaving many consumers — often those buying at lower prices — without comparable protection.
“It’s something in our psyche,” says John Warner, president of the Warner Babcock Institute for Green Chemistry, of the American predilection for deferring to marketplace rather than government solutions. 
Options and Solutions
Consumer demand and concern, often from mothers worried about the implications of certain chemicals for children’s health, has effectively pushed certain products — such as baby bottles made with bisphenol A — off the market. Such action is harder to effect with pesticides, but public outcry has been instrumental in moving the U.S. away from use of DDT and other such chemicals. Currently, public awareness of neonicotinoids’ adverse effects on bees has been raised dramatically by pollinator health advocacy campaigns. Actually shifting the agricultural market away from these products is a more difficult proposition. While the EU has promulgated policy using the precautionary principle and called a temporary halt to some of these pesticides’ use, the EPA is slowly continuing its review of these products — while at the same time approving new pesticides also toxic to bees.
What such an approach does not include is any guarantee of safer alternatives. Neither TSCA nor FDA regulations include such provisions. Many recently passed U.S. state chemical regulations, including California’s Safer Consumer Products program, have been written to address this concern, with language specifying that replacements for restricted chemicals be without adverse environmental health effects. That U.S. federal policies do not require as much pre-market information about chemical used in consumer products as does the EU system, adds to the difficulty of choosing safer alternatives.
When it comes to determining chemical safety of a consumer product, Warner sees fundamental flaws in the current approach. Restriction of hazardous chemicals in the U.S., EU and elsewhere — and in most corporate policies — is based on lists of chemicals of concern. By focusing on these lists, explains Warner, we fail to consider those chemicals not listed, a process that leads to what’s often referred to as regrettable substitutions. Instead, Warner advocates testing whole finished products and scoring them for health effects. Does a product exhibit carcinogenicity? Is it a neurotoxicant? Does it produce birth defects or adverse hormonal effects? Answering these questions would yield safer products more efficiently and effectively than our current system, says Warner, and would yield data that could be used objectively.
Screening methods that incorporate a comparable approach to rating chemicals’ toxicity by health endpoint, such as the non-governmental organization Clean Production Action’s GreenScreen, are now being used by many companies to assess individual chemicals. Warner argues that looking at whole finished products through this lens would help flag problematic chemicals not previously singled out for scrutiny, whether they are long-used existing compounds or brand new materials such as those he and other green chemists are now formulating.
So what’s the bottom line? Again, it’s complicated. When it comes to manufactured products such as computers and cosmetics, the global marketplace is playing a big role in turning one jurisdiction’s more stringent standards into industry standards because it’s often too costly to make different versions of the same product for different markets. Similarly, individual U.S. state policies restricting chemicals not regulated comparably at the federal level have motivated companies to respond with new formulations that end up being sold nationwide. At the same time, built into the U.S. chemical regulatory system is a large deference to industry. Central to current U.S. policy are cost-benefit analyses with very high bars for proof of harm rather than a proof of safety for entry onto the market. Voluntary measures have moved many unsafe chemical products off store shelves and out of use, but our requirements for proof of harm and the American historical political aversion to precaution mean we often wait far longer than other countries to act.
Shifting policy, particularly in a way such as Warner advocates, is perhaps an even slower proposition. But as Stacy Malkan points out, consumer demand for safe products isn’t going away any time soon.




Please talk about TTIP ! With it in EU we will have more chemical and less restriction.
Thank you for the eye opening article!
Fight on!
Please note that Pepsi & Coca-Cola (along with their sports drink lines, Powerade & Gatorade, and any products made by them) have discontinued BVO in any of their products as of May 2014. However, BVO is still legal in the U.S.