
Reports have recently surfaced that the EPA plans to raise the allowable radioactivity levels in drinking water by 3,000 times, which is the equivalent of receiving over 250 chest x-rays in one year. The notion that there is a safe level of radiation has been perpetuated by the US government for generations to promote the idea that nuclear energy is safe, but of course the government’s true motives behind the use of nuclear technology has little to do with energy, and more to do with weapons of mass destruction.
So let’s cut to the chase and take a closer look at the controversy behind nuclear energy, because the US government’s reasons for supporting it is what this all stems from. The TedTalks segment below covers both sides of the argument well:
There are a few things readers need to know about Stewart Brand, the gentleman in the video who was pro-nuclear, and whom the TedTalks host spoke so highly of:
From 1987 to 1989, Brand worked for corporations such as Royal Dutch/Shell—an energy-for-profit company—as a “private-conference” organizer for the corporation’s strategic planners. In 1988 he joined the Board of Trustees at the Santa Fe Institute, an organization founded by George Cowan, who was an American physical chemist known for his participation in the Manhattan Project (for those who are unfamiliar with the Manhattan Project, see our List of Most Horrifying US Government Experiments).

The Santa Fe Institute receives funding from various sources including government and corporate. This is normal for many institutes, but in the case of the Santa Fe Institute, it’s also a little… thought provoking, considering some of the areas of research at the institute are: Evolutionary diversification of viral strains, interactions and conflicts in primate social groups (primal traits still held by humans), as well as structures and dynamics of species interactions that includes food webs.
These topics of study might not seem too ominous at first, except the institute also has an interest in researching the emergence of hierarchy and cooperation in the human species, (Cowan, 2010). Cooperation sounds cool, but when it follows the word “hierarchy,” and when you mix it all together with government and corporate interests, it doesn’t immediately paint a pretty picture.
In 1987 Brand wrote “The Media Lab: Inventing the Future at MIT.” MIT, or the Massachusetts Institute of Technology, is also known for receiving extensive funding from the government and corporations, and they work closely with other institutes such as the David H. Koch Institute for Integrative Cancer Research—David Koch being one of the infamous “Koch brothers.”
The name “Koch” is almost synonymous with Rockefeller and Rothschild in the corporate-world. In recent years, the oil-tycoon brothers have been known to invest millions of dollars into the US’s higher education system—introducing curriculums focused on capitalism and industry rather than helping the poor or protecting the environment—and they’re known to influence elections through “dark money” groups.
While Stewart Brand is no doubt an intelligent man who has probably made some positive contributions to society, his affiliations with questionable institutes, corporations focused on energy-for-profits, and government agencies whose interests very likely contradict the people’s, brings to question the validity of his expertise on the matter of nuclear energy, as it very well may be influenced by those who funded and mandated his research.
Related News: Inside the Koch Brothers’ Toxic Empire
It’s no secret that the US government originally claimed its interest in nuclear energy was to kill two birds with one stone; nuclear energy, yes, but nuclear weaponry was a focus as well. Now that the public’s opinion on the use of nuclear weapons is turning unfavorable, it is especially important to boost nuclear energy’s reputation in order to retain steady access to the technology.
The fact is, while government and corporate-funded scientists tout about the advantages of nuclear energy, independent researchers and US scientists who’ve basically “blown the whistle” claim there is no such thing as a safe dose of radiation. In the case of the Chernobyl fallout, government-funded scientists claimed that around 2,000 to 75,000 premature deaths would occur around the world over a 70-year period from the fallout, but independent and impartial scientists put the estimates in the hundreds of thousands. One such scientist who supported this claim and came forward on the dangers of radiation was Dr. John Gofman (now deceased) of the government-supported Lawrence Livermore Laboratory, (Gofman, 1990).

According to environmental activist, writer, lecturer and organizer, Lorna Salzman, the actual long-term effects of low-level radiation exposure has “consistently been downplayed, distorted or concealed by scientists, the nuclear industry and the government,” (Salzman, 2002). The notion that non-observable, long-term latent effects of radiation in low doses causes no damage is easy to promote by the government since the effects do not manifest themselves for years, so by the time an individual develops cancer (as an example), there’s no way to prove it was caused by the radiation exposure.
The main “advantages” of nuclear power preached by pro-nuclear energy scientists are; the small amount of uranium needed, as well as the small amount of space the spent uranium takes up once disposed of (this doesn’t account for radioactive water produced at plants, which is becoming a problem), and the fact that nuclear energy is supposedly “clean.” What these scientists don’t tell you is that the spent uranium that’s been disposed of will still have to be monitored by future generations thousands of years from now, and if a train or truck transporting those spent fuel rods were to crash, the area in which it crashed would be uninhabitable for roughly 10,000 years after the clean-up, (American Chemical Society, 2016). In order to understand why this is, you need to have a basic understanding of what Uranium is.

Uranium is a heavy metal that occurs naturally in three isotopes, the most common of which—and what’s used for nuclear technology—is Uranium-238 which has a half-life alone of 4.46 billion years, making it an astronomically dangerous substance to work with, and yet we’re playing with it like a child who found his father’s gun. Uranium-238 decomposes into lead, and it is this lengthy decomposition process that makes it highly radioactive and unstable due to its emission of three kinds of ionizing radiation, that is; radiation that breaks chemical bonds giving it the ability to damage or destroy living cells within the body for years after exposure.
To get an idea of the amount of energy held within uranium; when the US dropped the bomb on Hiroshima, “Little Boy,” the nuke responsible for the destruction, contained just one gram of uranium, however only 2% of it actually detonated. That means a piece of uranium about the size of a peppercorn was responsible for leveling an entire city.
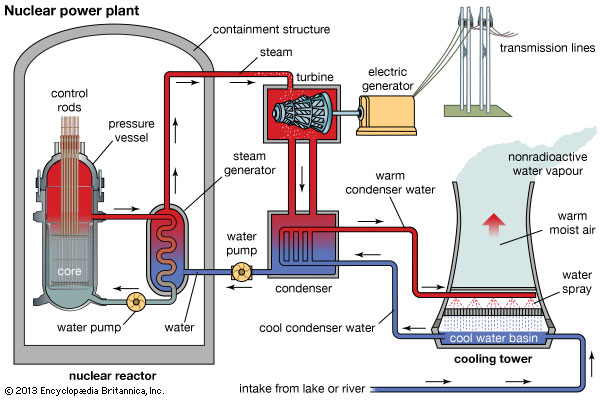
Now then, this is how nuclear fission at a power plant works:
The basic objective at a nuclear power plant is to heat water using energy from the uranium fuel rods in one chamber, and then circulating that heated (and highly radioactive) water through another enclosed (non-radioactive) water chamber to create steam. The steam transfers energy through a turbine, which then powers a generator, and from there the energy is transmitted to the public through power lines. It’s because of this use of steam that nuclear energy is considered “clean.”
Now notice the “control rods” located in the core; these rods, which are primarily composed of cadmium or boron, work to absorb either more, or less, of the extra neutrons emitted by the fuel rods, thereby keeping the fuel rods stable.

Phew, you made it through the lesson, now on to the point of this:
Even after a nuclear fallout occurs, the control rods that remain in any cores that were not destroyed still need to be constantly functioning and monitored to prevent the fuel rods within from causing another massive, radioactive explosion. The explosion at Chernobyl occurred in 1986, and yet the three remaining reactors stayed active years afterwards until the fuel rods could be disposed of, with the last reactor finally being shut down in 1999. Fuel rods in Fukushima aren’t planned to be removed until 2021, and the cleanup isn’t expected to end for another 30 to 40 years afterwards, however some nuclear specialists claim the plan is meant only to convince the public that Japan is recovering, and that it will take generations to even start the cleanup, and close to a hundred years to finish.
The news that the EPA plans to raise the allowed dosage of radiation should not only concern you, it should piss you off; there is nothing “safe” about ionizing radiation, and the only reason the public is being convinced otherwise is so the US government can keep hold of nuclear technology. One should ask themselves why nations such as India, who struggles with poverty, are spending billions of dollars investing in “nuclear energy” to rival the US when other forms of energy (that isn’t coal) is available.
Yes, bring on the arguments that wind takes up too much space, or geothermal is too expensive, but any reputable scientist will tell you that breakthroughs in energy technology will happen as soon as ten years from now, and if the focus is on truly clean forms of energy, that is where the breakthroughs will happen. If a nation such as India has more than $100 billion to spend on an energy plan, it’s worth further researching the alternatives in light of the dangers that accompany nuclear technology.
But let’s be realistic; it’s never been about energy.
This Article (Radioactive Drinking Water in the U.S. Raises the Nuclear Debate) is a free and open source. You have permission to republish this article under a Creative Commons license with attribution to the author and AnonHQ.com.
Sources:
American Chemical Society (2016). Chemistry in Context, (7th ed.). McGraw-Hill.
George A. Cowan (2010). Manhattan Project to the Santa Fe Institute: The Memoirs of George A. Cowan. Albuquerque, NM: University of New Mexico Press.
Gofman, John W. (1990). “Assessing Chernobyl’s Cancer Consequences: Application of Four `Laws’ of Radiation Carcinogenesis. Paper presented at the Symposium on Low-Level Radiation, National Meeting of the American Chemical Society, September 9, 1986.
Salzman, Lorna (2002). Pro-Nuclear Propaganda: How Science, Government and the Press Conspire to Misinform the Public. Lecture delivered by Lorna Dalzman at Hunter College, Energy Studies program, 1986. Updated 2002. Retrieved from: http://www.lornasalzman.com/collectedwritings/pro-nuclear.html






Where Did Natural Background Radiation Come From?
The sum of the natural background radiation at Fukushima plus the radiation leak from the reactor is less than the natural background radiation where I live in Illinois. There was no reason for Japan to shut down their reactors. If the reactors at Fukushima had not been shut down, would they have continued to operate normally?
Where did natural background radiation come from? The universe started out with only 3 elements: hydrogen, helium and lithium. All other elements were made in stars or by supernova explosions. Our star is a seventh generation star. The previous 6 generations were necessary for the elements heavier than lithium to be built up. Since heavier elements were built by radiation processes, they were very radioactive when first made.
Our planet was made of the debris of a supernova explosion that happened about 5 billion years ago. The Earth has been decreasing in radioactivity ever since. All elements heavier than iron were necessarily made by accretion of mostly neutrons but sometimes protons onto lighter nuclei. Radioactive decays were necessary to bring these new nuclei into the realm of nuclear stability. That is why all rocks are still radioactive.
Radiation also comes from outer space in the form of cosmic rays. Cosmic rays come from supernovas that are very far away. There will always be cosmic rays.
The problem is that the Japanese people did not measure the natural background radiation before 1940, so they don’t know that they are trying to get rid of the natural background. It is not possible to get rid of the natural background.
Wow. I’ll have to do some research on this. I’ve never heard that information and it interests me greatly. Thanks for sharing.
Nuclear power is the only way to stop making CO2 that actually works. To stop Global Warming, we must replace all large fossil fueled power plants with nuclear.
Renewable Energy mandates cause more CO2 to be produced, not less, and renewable energy doubles or quadruples your electric bill. The reasons are as follows:
Since solar “works” 15% of the time and wind “works” 20% of the time, we need either energy storage technology we don’t have or ambient temperature superconductors and we don’t have them either. Wind and solar are so intermittent that electric companies are forced to build new generator capacity that can load-follow very fast, and that means natural gas fired gas turbines. The gas turbines have to be kept spinning at full speed all the time to ramp up quickly enough. The result is that wind and solar not only double your electric bill, wind and solar also cause MORE CO2 to be produced.
We do not have battery or energy storage technology that could smooth out wind and solar at a price that would be possible to do. The energy storage would “cost” in the neighborhood of a QUADRILLION dollars for the US. That is an imaginary price because we could not get the materials to do it if we had that much money.
The only real way to reduce CO2 production from electricity generation is to replace all fossil fueled power plants with the newest available generation of nuclear. Nuclear can load-follow fast enough as long as wind and solar power are not connected to the grid. Generation 4 nuclear can ramp fast enough to make up for the intermittency of wind and solar, but there is no reason to waste time and money on wind and solar.
Spent nuclear fuel is not a proliferation risk because a power plant makes the wrong isotopes of plutonium for bombs. To make a good bomb, you need pure plutonium239 [Pu239].
Isotopes: Any chemical element can come in several isotopes.
To make Pu239, you have to shut down the reactor and do a fuel cycle after one month or less of operation. Since removing and replacing fuel takes a month, a short-cycled reactor operates half the time. A power plant that has a one month on, one month off fuel cycle would stick out a lot more than the proverbial sore thumb.
A reactor used to make electricity runs for 18 months to 2 years between refuelings. An individual fuel rod will stay in the reactor for 3 cycles since only ⅓ of the fuel rods are exchanged at each fueling, so one fuel rod stays in the reactor 4.5 to 6 years. In that time, many trans-uranic elements are created. In that time, Pu239 absorbs extra neutrons, becoming Pu240, Pu241, Pu242, 95americium243, 96curium247, 97berkelium247, 98californium251, 99einsteinium25, 100fermium257 and so on.
All of these higher actinides are good reactor fuel but bad for bomb making. Bombs made of spent fuel have been made and tested once or twice [US and North Korea]. They pre-detonate and fizzle so badly that a very large conventional bomb can equal the yield. They are so radioactive that a poor country can’t build one without killing the scientists. They are militarily worse than useless [Till & Chang book “Plentiful Energy”]. There is no country that has a spent fuel bomb, nor will anybody build one in the future. An insane person trying to build one would die a few seconds to minutes after having acquired the spent fuel.
7% Pu240 is enough to spoil a bomb and you get a lot more than 7% Pu240 from a reactor that has been running for 18 months. Separating Pu239 from those higher actinides is a technology that has not been developed. Nobody would try to do that separation because the easy way to make Pu239 is with a short cycle reactor. Governments that have plutonium bombs, have government owned government operated [GOGO] reactors that do nothing but make Pu239.
Mark Jacobson is telling lies.
The author of this article displayed his ignorance in several ways. For one thing, the NRC has NOT raised radiation limits to the equivalent of 250 chest X-rays. (It is the Nuclear Regulatory Commission which regulates radiation exposure, not the EPA.) The maximum annual exposure remains 50,000 micro-Sieverts. Second, U238 is NOT the “active” isotope of uranium. The fissile isotope is U235. Third, because the half life of U238 is 4.468 BILLION years, it is not a radiation hazard. Saying it is shows an incredible lack of understanding of what half life means. Materials which are hazardous have very SHORT half lives. In other words, half of the atoms decay each half life. So a short half life means lots of decays with lots of accompanying radiation. Materials with very long half lives have very few atoms decaying in a given year and thus produce very little ionizing radiation. I could go on, but just these glaring errors should indicate the ignorance, fear mongering, and lack of quality reporting in this article.
The EPA has raised the limits in case of an incident. This is actually true, Gary. The half-life of uranium is dangerous due to the fact it can contaminate an area for thousands of years. It’s “time” here which is the specified danger, not a correlation between the amount of radioactivity and the half-life. Heavy elements such as Uranium (of any isotope) have a stronger electrical barrier around its nucleus, and releases this binding energy through gamma rays (ionizing radiation). Uranium-238 IS used in nuclear technology, the article was correct on that, and despite the fact Little Boy used Uranium-235, its still important for people to have an idea of how much energy can be held within this element. How better to give the reader an idea then by telling them how much uranium it took to blow up a city? I think Astroturfer raised a good question. What agency do you work for?
what agency do you work for?
Hydro… Look it up, Qc, CA.